Theme: Growth & Future of Pharmacovigilance & Drug Safety
Pharmacovigillance 2022
Conference series is organizing splendorous Pharmaceutical conferences welcomes you to attend the " 15th Annual Meet on Pharmacovigilance & Drug Safety” to be held during November 21-22, 2022 London, UK. This Conference Provides a forum of interaction focuses advancement of risk factor. It focuses on the advancements in Pharmacovigilance, Risk Management, Toxicology and Drug Safety. The field of Pharmacovigilance is growing quickly and its development is creating tremendous impacts in medical sciences and prescribed drugs. 15th Annual Meet on Pharmacovigilance & Drug Safety emphasizes on however the importance and significance may be gauged by the actual fact that it's created immense advancements over the course of time and is constant to influence varied sectors. The theme of this year’s meeting is " Growth & Future of Pharmacovigilance & Drug Safety".
The Field of Pharmacovigilance growing rapidly now a days & development is making impact on pharmaceutical Science. In the light of this theme, the Conference series aims to provide Detection & evaluation of drug Safety Signals, Data Quality management. It involves all activities that relate to noticing, assessing, understanding, managing and preventing adverse effects of medicines for individuals and populations
What’s New?
15th Annual Meet on Pharmacovigilance & Drug safety 2022 offers a best stage with its proficient legitimate program to the social event of individuals which consolidates instinctive board discoursed, keynote addresses, all-hands gatherings and notice sessions on the topics Pre-Clinical and Clinical Trials, Adverse Drug Reactions, Pharmacovigilance and Risk Management, Good Pharmacovigilance Practice, Pharmacy Practices and its Challenges, Biopharmaceutical Sciences, Clinical Trials on Various Disorders, Data Quality Management and Analysis, Pharmacovigilance Significance & Scope, Diversity in Industrial Clinical Trials and Clinical Research, Clinical Research and Statistics, Case Report in Clinical Trials, Drug Safety, Clinical Data Base Management, Regulatory Affairs, PV Consulting’s and Business Opportunity. Entrepreneurs Investment Meet. This is your best opportunities to reach the largest participants from pharmacovigilance community. Conduct presentation, meet with scientist, making a new drug development, world renewed Speakers, Clinical trials. This conference is a 2 days event.
Target Audience
Clinical Laboratories and Technicians
Pharmaceutical Industrial Giants.
Research Institutes and members.
Nursing Professionals
Pharmacovigilance Researchers
Pharmacovigilance Faculty
Medical Colleges
Pharmacovigilance Associations and Societies
Training Institutes
Software Developing Companies
Manufacturing Medical Devices Companies
Track.1 Pharmacovigilance and Clinical Trials
Pharmacovigilance and Medical Trials will have the fast progress of new drug and innovative therapeutics, new pharmacovigilance actions and methods have to be applied for guarantee and Patient Advantage Safety Organization in Pharma, Biotech and Health Care. Clinical Traces contain the best excellence level to estimate the viability and welfare of new medications. However, in light of the fact that they are focused in homogeneous conditions a long way from this current reality of answer and use, errors in patient choice or conduct situations may change both the possibility and risks.
Track.2. Drug Safety
The drug safety thought has got plenty of attention during the past period cheers to the very fact it plays a key role in patients’ health. Latest laws worry this idea must to be enclosed within the process of latest medications’ support and constant behavior of post-marketing drug evaluations. Advantage–risk calculation must to be imaginary of by all fitness care specialists once they ought to offer careful medication to detailed teams of patients. Therefore, additional care ought to slope to some patients, like pregnant girls, youngsters and therefore the aged, meanwhile they're thought of susceptible populations.
Track.3. Pharmaceutical Chemistry
Pharmaceutical chemistry is the study of medicine and it includes drug improvement. This contains drug discovery, delivery, absorption, metabolism, and more. There are the basics elements of biomedical study, pharmacology, pharmacokinetics and pharmacodynamics. Pharmaceutical chemistry work is usually wiped out a laboratory setting. Pharmaceutical chemistry contains cures and remedies for disease, analytical techniques, pharmacology, metabolism, quality assurance, and drug chemistry.
Track.4. Clinical Research and Statistics
Clinical study contains exploring deliberate medicinal behaviors, evaluating the relative benefits of good therapies, and opening optimum action combos. Clinical analysis varieties and effort to answer questions like “should a person with glandular carcinoma bear, radical ablation or radiation or wait and see?” Digits play an awfully significant role in any path from style, conduct, analysis and reportage in terms of main for and minimizing biases, clashing factors, and mensuration irregular errors.
Track.5. Pharmacovigilance Practice
GVP is abbreviated as pharmacovigilance practices which is to enable to measure and presentation of Pharmacovigilance. The role of GVP and Pharmacoepidemiology in Risk Management is to raise the useful effects of a drug than its opposing effects. The scientific trials and pharmacovigilance amenities providing companies must have Certification. It is of high rank to focus on Signal investigation via observational studies to understand safety signals.
Track.6. Adverse Drug Reactions
These reactions are an open trial for Healthcare professionals -to know the problem and be aware of how these reactions can be stopped and managed. However, opposing drug responses can be reduced including the patient as one pillar of the healing plan and providing more patient therapy, which will recover drug safety. Evaluating and reporting adverse drug reactions are very significant to uphold drug safety systems. PK-PD studies are the two major focus areas of pharmacology.
Track.7. Clinical Trials on Various Disorders
Clinical trials are nothing but research done in clinical study. Clinical investigation method includes a compound network of pharmaceutical companies, sites and educational study institutions/ laboratories. Each clinical trial has a strategy of action or a procedure for directing trial. Clinical trials produce data on security and efficacy. Here in these track discussions are involved in types of diseases and theirs clinical test output.
Track.8. Pharmacy Practices and its Challenges
In Pharmacy Practices and its Trials track principally targeted on Pharmacy observe and its pointers and Trials in change of integrity and supply observe. Indefinite measure program, drug toxicity and drug safety measures place vital place in scientific study. Approaches for Growth in pharmaceutical company atmosphere are targeted in Strategic growth towards bureau support and Post marketplace product Surveillances.
Track.9. Clinical Database Management
The field of clinical knowledge management (CDM) has recognized itself cheers to demands from every the pharmaceutical business and therefore the preventive authorities. In the meantime the originality to “fast-track” the period of pharmaceutical product stands to rush, preventive objects have replied by needful quality-assurance values be chanced in accruing the info active in the drug analysis system.
Track.10. Analysis of Data Quality and Management
Pharmacovigilance depends on data collected from the gathering of separate case protection reports and unlike pharmacoepidemiological information. Even the vital limits of impulsive reports, the merit of this evidence supply will be improved with smart information worth management. Though under-reporting cannot be cured this manner, the destructive impact of unfinished reports, that is another important issue in pharmacovigilance, will be abridged.
Track.11. Drug related problems in healthcare
Pharmacotherapy has been related with undesirable health consequences such as adverse effects, interactions, adherence problems, functional decline, cognitive problems, falls, urinary incontinence and metabolic or nutritional problems6,7,8,9,10,11,12,13. The risk of these problems increases with the number of drugs. Polypharmacy, defined as the use of more than four or five drugs, occurs in 40% of the adults over 65 years old.
Track.12. Pediatric Pharmacy
Pediatric drug experts guide children and their parents about clarification and write treatments if needed. They track their patients' therapeutic growth and safeguard that there are no harmful drug relations or unexpected side effects. Pediatric drug specialists frequently work in healing offices like specialist's offices, drug stores, and healing facilities.
Track.13. Pharma Medicinal Chemistry
Medicinal chemistry and pharmaceutical chemistry are controls at the merging of science, mostly caused natural science, and pharmacology and unlike other organic claims to fame, where they are included with plan, complex union and growth for market of medical specialists, or bio-dynamic particles. Disclosure is the recognizable proof of novel dynamic concoction mixes, often called "hits", which are commonly found by test of mixes for a imaginary natural action.
Track 14. Pharmacognosy and Phytochemistry
Pharmacognosy, a long settled pharmaceutical science, has assumed a various part in the disclosure, characterization, generation and institutionalization of these medications. The importance of this train regarding examination and instructing has expanded in the most recent decade as individuals from the general population in created nations have swung to the utilization of home grown solutions for the self-medicine of minor ailments. In any case, numerous phytomedicines require advance examination for their clinical adequacy, while others should be altogether researched for their potential wellbeing dangers or connections with physician recommended drugs.
Phytochemistry is naturally huge by assuming a basic part in the plants to guard themselves against different pathogenic microorganisms by demonstrating the antimicrobial action by hindrance or executing systems. The discharge of these mixes is differing from plant to plant some deliver increasingly and some create in insignificant amount. Now and then they can be unsafe and some of the time they can be exceptionally useful.
The global pharmacovigilance (PV) market is expected to reach USD 10.27 billion by 2025, according to report by Grand View Research, Inc. The market is expected to witness growth at 13.1% CAGR owing to Increasing incidence of ADR is key driver for the growth of pharmacovigilance market.
According to a report by Grand View Research, Inc.; the global Pharmacovigilance (PV) market is projected to reach USD 10.27 billion by 2025. Rising cases of Adverse Drug Reactions (ADRs) are anticipated to drive the market during the forecast period (2018 to 2025). Rising consumption of combination of drugs owing to increasing cases of chronic disorders can result in ADRs. This factor is anticipated to augment demand for pharmacovigilance services over the forecast period. Growing number of nonprofit organizations such as International Society of Pharmacovigilance (ISoP) to promote benefits of PV services can further impel market growth.
Supportive government initiatives to promote the use of pharmacovigilance services can drive market growth over the forecast period. Increasing use of medicines to treat chronic diseases is likely to account for a huge proportion of overall consumption of drugs in hospital set-ups. This factor is projected to boost demand for medicines among healthcare providers, thereby driving the need for development of novel therapeutics through extensive clinical trials. Moreover, growing R&D activities in major pharmaceutical companies to develop innovative therapeutic drugs can further boost market growth over the forecast period.
U.S. pharmacovigilance market revenue by clinical trial phase, 2014 – 2025 (USD million)
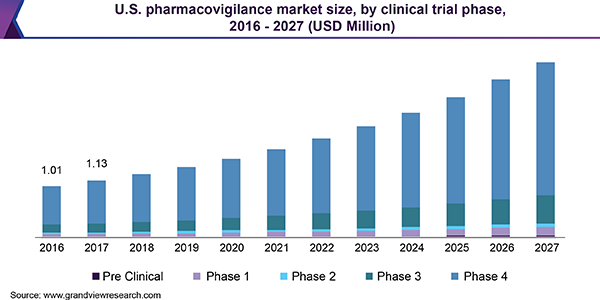
The worldwide Pharmacovigilance (PV) market can be segregated on the basis of clinical trial phase, service provider, type, end-use, and region. Based on clinical trial phase, the market can be categorized into phase 1, phase 2, phase 3, phase 4, and pre-clinical. In 2016, phase 4 segment dominated the market. PV solutions act as additional safety feature for drugs in clinical trials. Phase 4 is estimated to be important stage of clinical trials attributed to detection of unpredicted adverse drug reactions during this stage. Hence, the data monitored and collected during this stage is considered of high relevance.
Based on service provider, the market can be bifurcated into contract outsourcing and in-house. In 2016, contract outsourcing segment dominated the market and is anticipated to expand at the fastest CAGR in the forthcoming years. Benefits associated with PV services including resource flexibility and reduction in fixed cost, risk, and upfront investments are projected to drive the demand over the forecast period.
Conference Series LLC ltd is a renowned organization that organizes highly notable conferences is successfully affixed its “13th Global Pharmacovigilance & Clinical Trials Summit” (Global Pharmacovigilance-2019) slated during March 28-29, 2019 at Hotel Agora Moriguchi in Osaka, Japan.
Our “13th Global Pharmacovigilance & Clinical Trials Summit” was based on the theme of “Promulgating the prevention of adverse drug reaction” which has covered the below scientific sessions:
- Clinical Research and Statistics
- Adverse Drug Reactions
- Causality Assessment
- Hospital and Industrial Pharmacy
- Clinical Trials Pharmacovigilance
- Pharmacovigilance Significance & Scope
- Pharmacovigilance Practice
- Drug Safety
- Clinical Trial Protocols
- Diversity in Industrial Clinical Trials and Clinical Research
- Clinical Database Management
- PV Consulting’s And Business opportunity
- Bio pharmaceutics
- Regulatory Affairs
- Analysis of Data quality and Management
- Entrepreneurs Investment Meet
The Conference received acknowledgments & immense support was extended by the keynote lectures mentioned below:
- Jacob Joseph, Howard Medical School, USA
- Ujwala Vilas Salvi, Nucleon Therapeutics LLP, Mumbai (India)
- Chi Chen, Partner at E&Y,CHINA
- Rajiv Joshi, Director and Assuranc. ,E &Y
- Carole Gabay¸ DRcomGroup ,China
- Fawad Piracha, Regeneron Pharmaceuticals, Inc ,USA
Conference Series LLC Ltd wishes to acknowledge with its deep sincere gratitude to all the supporters from the Editorial Board Members of our Open Access Journals, Keynote speakers, Valuable speakers, students, delegates for their contribution to make this event a huge success.
We once again thank you all for the enormous exquisite response. This inspires us to continue organizing events and conferences for furthering the pharmacovigilance Research. Conference Series LLC ltd therefore, is glad to announce its Annual Meet on Pharmacovigilance & Drug Safety scheduled during November 21-22, 2022 London, UK.
Conference Highlights
- Pharmacovigilance and Clinical Trials
- Drug Safety
- Pharmaceutical Chemistry
- Clinical Research and Statistics
- Pharmacovigilance Practice
- Adverse Drug Reactions
- Clinical Trials on Various Disorders
- Pharmacy Practices and its Challenges
- Clinical Database Management
- Analysis of Data Quality and Management
- Drug related problems in healthcare
- Pediatric Pharmacy
- Pharma Medicinal Chemistry
- Pharmacognosy and Phytochemistry
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | November 21-22, 2022 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmacovigilance
- International Journal of Pharmacovigilance
- Jacobs Journal of Pharmacology and Pharmacovigilance
Abstracts will be provided with Digital Object Identifier by














